Valve body corrosion is a significant challenge for industries such as oil and gas, water treatment, chemical processing, marine applications, and general industrial use. Corrosion not only affects valve performance and safety, but can also cause significant economic losses due to downtime, repair, or replacement. This newsletter will take a deep dive into the types of corrosion commonly encountered in these industries, effective protection methods, and strategies to extend valve life.
1. Understanding Valve Body Corrosion
1.1 What is corrosion?
Corrosion is a natural process during valve use, where metal degrades due to reaction with the environment. This can cause leaks or failure.
Corrosion occurs primarily when valve materials react with oxygen, moisture, or chemicals, causing the structural integrity to be compromised.

1.2 Types of Valve Body Corrosion
Valve bodies are exposed to a variety of environmental and process conditions, resulting in different types of corrosion.
1.2.1 Pitting

Pitting is a localized corrosion that causes small, deep cavities or “pits” in the metal surface. Pitting occurs only in specific areas rather than uniformly corroding the entire surface. It is often triggered by chloride ions and is particularly harmful because it is difficult to detect and can cause structural failures that affect the seal.
The most difficult feature of pitting is that it is difficult to detect by visual inspection and may cause failure without obvious appearance damage. And once formed, pitting usually spreads rapidly.
Common causes
Chloride ions (Cl⁻): Common in seawater, chemical solutions or water treatment systems, fluids containing chloride ions have a much higher conductivity rate than fresh water.
Differences in oxygen concentration: Initiate local redox reactions.
Metal surface defects: Such as microcracks or coating damage are easy to become the starting point of pitting.
1.2.2 Stress Corrosion Cracking (SCC)

Stress corrosion cracking is a metal cracking phenomenon caused by the combined action of tensile stress and corrosive environment on certain alloy component materials.
For butterfly valves, stress corrosion cracking is common when certain materials (such as austenitic stainless steel) work in chloride-containing environments or high temperatures.
For example, in oil and gas applications, oil and gas production systems containing hydrogen sulfide (H2S), low pH values and reduced redox potentials create conditions that are favorable for the formation of atomic hydrogen. This hydrogen penetrates into the corroded anode locations within the alloy. As hydrogen atoms accumulate in these areas, internal pressure increases, and once a critical threshold is reached, energy is suddenly released.
Compared to pitting, stress corrosion cracking develops relatively slowly, and cracks usually start from tiny surface defects or stress concentrations and gradually expand.
The resulting damage manifests as cracks within the alloy, which are usually formed internally and cannot be detected on the surface. Under tensile stress, these cracks will expand, greatly reducing the ductility of the alloy.
In addition, stress corrosion cracking (SCC) causes alloys that are usually ductile to exhibit brittle behavior under certain conditions.
1.2.3 Erosion corrosion

Erosion corrosion is common in water treatment plants and chemical processing industries. It is caused by the combined effects of mechanical wear and chemical attack, and is usually caused by damage to the metal surface in an environment where high-speed fluids or media are mixed with solid particles (such as mud) or bubbles are present.
1.2.3.1 Causes of erosion corrosion
* Scouring of high-flow high-speed fluids
When the fluid flows at high speed in the valve body, it produces shear stress on the metal surface, which can cause the protective layer to fall off over a long period of time, thereby inducing corrosion.
* Impact of solid particles
When the medium contains sand or other hard abrasive particles, these particles will hit the inner wall of the valve body at high speed, causing surface wear and accelerating the chemical corrosion process.
* Bubbles and cavitation
When the fluid pressure is reduced, the liquid may vaporize to form bubbles. When these bubbles burst in the high-pressure area, they will release energy and have a strong impact on the valve body surface, forming cavitation corrosion.
1.2.3.2 Common locations of erosion corrosion
* Valve body inlet and outlet
The drastic change in fluid velocity can easily cause turbulence, which in turn has a strong impact and corrosion on the metal surface of these areas.
* Flow channel corners and narrow parts
When the fluid flows through corners or throttling areas, local high flow velocities will be formed, increasing the risk of erosion corrosion.
* Joint components and sealing surfaces
Due to the dual effects of long-term mechanical stress and chemical corrosion, these areas are prone to material peeling or deformation.
2. Valve Body Corrosion Protection Methods
2.1 Coatings for Valve Body Corrosion Protection
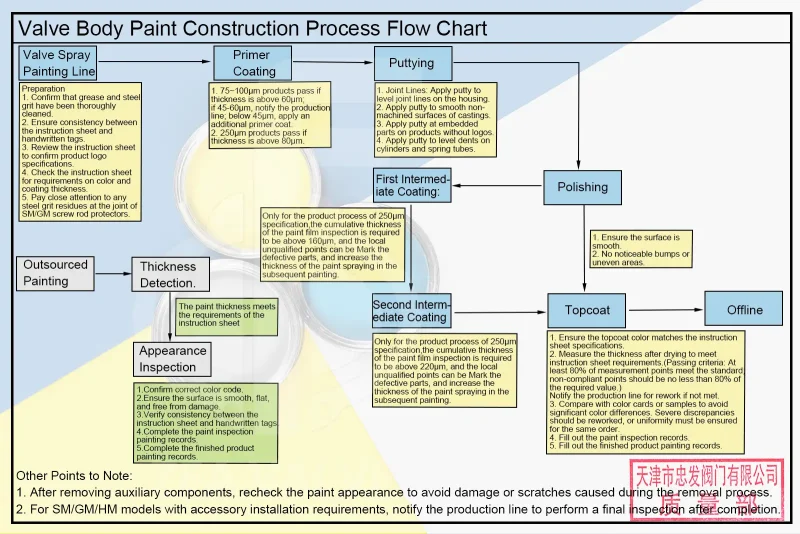
Coatings are the first protective barrier between valve materials and their environment. They are one of the most common and widely used anti-corrosion methods.
Paint construction process:
a) Preparation work:
including cleaning the surface grease and steel sand, scraping putty to fill the box joint line, non-machined surface of castings, product inserts without LOGO, and depressions on the surface of cylinder barrels and spring barrels, etc.
b) Primer construction:
test the primer thickness according to product specifications, and rework or re-spray if unqualified.
Intermediate paint construction: polish until the surface is smooth without obvious bumps, test the paint film thickness and mark the defective parts.
c) Topcoat construction:
confirm that the color number is consistent with the instruction sheet, measure the paint film thickness after drying, and notify the production line to rework if unqualified.
d) Paint technical specifications:
C4, C5 environment: use epoxy zinc-rich primer, epoxy micaceous iron paint a) intermediate paint and aliphatic polyurethane topcoat, and the specific ratio and use time have clear requirements.
e) C3 general environment:
use epoxy primer and aliphatic polyurethane topcoat or water-based polyurethane topcoat, and the ratio and use time are implemented according to the specifications.
Surface treatment: sandblasting to SA2.5 level to ensure that the surface is clean, dry and oil-free.
f) Construction precautions:
Construction is not recommended when humidity is greater than 85% or on rainy days.
After mixing the main paint and curing agent, stir evenly and mix as much as needed.
The amount of thinner is adjusted according to the amount of paint, temperature and climate environment, and spraying equipment.
After the inorganic silicate zinc-rich primer is completely dry, the intermediate paint needs to be sprayed with mist.
2.1.1 Epoxy coating

Epoxy coatings are durable and chemically resistant and are often used in water treatment and chemical processing. They have excellent adhesion and long-lasting protection.
2.1.2 Polyurethane coatings

These Polyurethane coated butterfly valves have excellent wear resistance and are suitable for marine and industrial applications.
2.1.3 Ceramic coatings
Ceramic coatings are ideal for high temperature and chemically corrosive environments, with extremely high durability to prevent wear and chemical erosion.
2.1.4 Galvanized coatings
These coatings act as sacrificial layers to prevent corrosion of the underlying material. The galvanized butterfly valve are commonly used in oil and gas applications.
2.2 Cathodic Protection for Valve Body Corrosion Protection
Cathode protection is an electrochemical corrosion protection technology that prevents oxidation reactions (i.e. corrosion) by changing the electrochemical behavior of metals.
The basic principle of cathodic protection is:
Sacrificial anode protection: By installing sacrificial anodes (such as materials such as zinc, magnesium or aluminum) near the butterfly valve body, these anodes will be corroded preferentially, while the butterfly valve body is protected. The sacrificial anode absorbs the corrosion current through electrochemical reaction with the valve body, reducing the corrosion of the valve body itself.
Impressed current protection: In some applications, an external power supply applies current to the valve body, forcing the valve body to become a cathode and inhibiting its corrosion. This method requires the installation of a current source and connecting electrodes, and is suitable for valves exposed to strong corrosive environments for a long time.
Cathodic protection involves the use of sacrificial anodes or impressed currents to transfer corrosion from the valve body.
2.2.1 Sacrificial anode system
Material such as zinc, aluminum or magnesium acts as an anode and corrodes instead of valve materials.
2.2.2 Impressed current system
An external power supply provides current to counteract the corrosion reaction. This method is particularly effective in large applications such as pipelines and offshore platforms.
2.3 Material selection
Choosing the right material is essential to prevent corrosion. Advanced alloys and non-metallic materials have excellent corrosion resistance in specific environments.
2.3.1 Corrosion Resistant Metals

• Stainless Steels: Resistant to pitting and SCC, especially when alloyed with molybdenum.
• Duplex Stainless Steels: Combine strength and corrosion resistance for use in harsh environments.
• Inconel and Hastelloy: High-performance alloys for use in extreme chemical conditions.
2.3.2 Non-Metallic Materials
• PTFE (Polytetrafluoroethylene): Chemically inert, suitable for lining valve interiors.
• Thermoplastics: Resistant to specific chemical exposures in water and wastewater applications.
2.4 Corrosion Inhibitors
The valve body corrosion protection process can be slowed or prevented by adding inhibitors to the fluid media or applying them directly to the valve body surface.
2.4.1 Types of Inhibitors
• Anodic Inhibitors: Inhibit the anodic reaction (metal oxidation process) by forming an oxide film on the metal surface. Common Inhibitors: Chromates, Nitrates, Phosphates, etc. Among them, our Our most commonly used phosphating body butterfly valve.

• Cathodic Inhibitors: Inhibit corrosion by slowing down the cathodic reaction (oxygen reduction or hydrogen reduction). Common Inhibitors: Zinc Salts, Barium Salts, Polymers, etc.
• Mixed Inhibitors: Act on both the anode and cathode to provide comprehensive protection. Common inhibitors: amines, nitrites, organic phosphonic acids, etc.
• Vapor phase corrosion inhibitors (VCI): After volatilization, a protective film is formed to cover the metal surface to prevent corrosion. Common inhibitors: nitrosamine, cyclohexylamine, etc.
• Organic corrosion inhibitors: By adsorbing on the metal surface to form a protective film, the corrosive medium is inhibited from contacting the metal. Common inhibitors: imidazoles, mercaptans, alcohol amines, etc.
2.4.2 Applications
Inhibitors are widely used in closed systems, such as boilers and cooling circuits, as well as pipelines and tanks.
3. Design Considerations for Valve Body Corrosion Protection
3.1 Avoid gaps
Designing valves with minimal gaps and smooth surfaces can help reduce the risk of crevice corrosion.
3.2 Flow optimization
Minimizing turbulence and ensuring uniform flow distribution can reduce erosion corrosion.
3.3 Selecting compatible materials
Use materials with similar electrochemical properties to prevent electrochemical corrosion.
4. Industries and applications affected by corrosion
4.1 Oil and gas
Valves in this industry are used to handle corrosive media, including hydrocarbons, sour gases, and high-pressure fluids. Corrosion can compromise safety and cause environmental harm.
4.2 Water Treatment
Valves are exposed to chlorinated water, salt, and other chemicals that promote pitting and crevice corrosion. Effective corrosion protection ensures a reliable water supply.
4.3 Chemical Processing
Chemical plants handle acids, bases, and other corrosives, requiring strong protection to prevent material degradation.
4.4 Marine Applications
Salt water is highly corrosive, especially to ferrous metals. Corrosion protection is critical for valves used on ships, offshore platforms, and coastal facilities.
4.5 General Industrial Uses
Industries such as power generation and manufacturing require valves that can resist a variety of corrosion, depending on their specific applications and environments.
5. Maintenance Practices to Mitigate Corrosion
5.1 Periodic Inspections
Performing visual and nondestructive testing (NDT) can help identify early signs of corrosion.
5.2 Cleaning and Flushing
Regular cleaning removes deposits and contaminants that can accelerate corrosion
5.3 Recoating and Repair
Promptly reapplying protective coatings and repairing damaged areas ensures continued protection.
6. Corrosion Standards and Certifications
6.1 Industry Standards
• NACE MR0175/ISO 15156: Corrosion-resistant materials for sour gas environments.
• API 609: Governs valve design and material specifications.
• ASTM G31: Standard for corrosion testing in laboratory environments.
6.2 Certification Bodies
Valves and coatings should meet standards set by organizations such as WRAS, DVGW, and NSF to ensure quality and safety.





